Funny Video Related to Coulombs Law and Newtons Law
Learning Objectives
By the end of this section, you will exist able to:
- State Coulomb's law in terms of how the electrostatic strength changes with the altitude between two objects.
- Calculate the electrostatic force between two charged point forces, such every bit electrons or protons.
- Compare the electrostatic forcefulness to the gravitational attraction for a proton and an electron; for a human and the Earth.
 Figure 1. This NASA image of Arp 87 shows the effect of a stiff gravitational allure between ii galaxies. In dissimilarity, at the subatomic level, the electrostatic attraction between 2 objects, such every bit an electron and a proton, is far greater than their mutual attraction due to gravity. (credit: NASA/HST)
Figure 1. This NASA image of Arp 87 shows the effect of a stiff gravitational allure between ii galaxies. In dissimilarity, at the subatomic level, the electrostatic attraction between 2 objects, such every bit an electron and a proton, is far greater than their mutual attraction due to gravity. (credit: NASA/HST)
Through the work of scientists in the tardily 18th century, the main features of the electrostatic strength—the existence of ii types of accuse, the observation that like charges repel, unlike charges attract, and the decrease of force with altitude—were eventually refined, and expressed as a mathematical formula. The mathematical formula for the electrostatic force is called Coulomb's law after the French physicist Charles Coulomb (1736–1806), who performed experiments and get-go proposed a formula to calculate it.
Coulomb's Law
Coulomb'south law calculates the magnitude of the force F betwixt two point charges, q i and q ii, separated by a distance r. In SI units, the constant 1000 is equal to
The electrostatic force is a vector quantity and is expressed in units of newtons. The force is understood to be forth the line joining the two charges. (See Effigy 2).
Although the formula for Coulomb's constabulary is simple, information technology was no hateful chore to prove it. The experiments Coulomb did, with the primitive equipment so available, were hard. Modern experiments have verified Coulomb'due south law to bully precision. For example, it has been shown that the force is inversely proportional to distance between two objects squared
to an accurateness of ane role in 1016. No exceptions have ever been constitute, even at the small distances inside the atom.
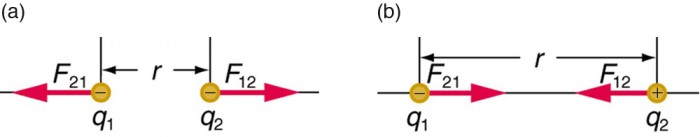 Figure 2. The magnitude of the electrostatic force F between indicate charges q 1 and q 2 separated by a altitude r is given by Coulomb'due south police force. Note that Newton's tertiary law (every strength exerted creates an equal and opposite force) applies as usual—the force on q i is equal in magnitude and reverse in direction to the strength it exerts on q 2. (a) Like charges. (b) Unlike charges.
Figure 2. The magnitude of the electrostatic force F between indicate charges q 1 and q 2 separated by a altitude r is given by Coulomb'due south police force. Note that Newton's tertiary law (every strength exerted creates an equal and opposite force) applies as usual—the force on q i is equal in magnitude and reverse in direction to the strength it exerts on q 2. (a) Like charges. (b) Unlike charges.
Example one. How Strong is the Coulomb Force Relative to the Gravitational Force?
Compare the electrostatic force between an electron and proton separated by 0.530 × x−x m with the gravitational force between them. This distance is their boilerplate separation in a hydrogen atom.
Strategy
To compare the two forces, we first compute the electrostatic force using Coulomb'south law,
. We and so calculate the gravitational forcefulness using Newton'south universal constabulary of gravitation. Finally, nosotros take a ratio to see how the forces compare in magnitude.
Solution
Entering the given and known information about the charges and separation of the electron and proton into the expression of Coulomb'south law yields
Thus the Coulomb forcefulness isF = 8.xix × 10−8 Due north.
The charges are contrary in sign, so this is an attractive force. This is a very large force for an electron—it would cause an acceleration of 8.99 × 1022 yard/due south2 (verification is left equally an end-of-section problem).The gravitational strength is given by Newton'south law of gravitation as:
,
where Thou = 6.67 × 10−xi N · thousand2/kgtwo. Here m and M represent the electron and proton masses, which can exist found in the appendices. Entering values for the knowns yields
This is also an bonny force, although information technology is traditionally shown every bit positive since gravitational force is always attractive. The ratio of the magnitude of the electrostatic forcefulness to gravitational force in this case is, thus,
.
Discussion
This is a remarkably large ratio! Notation that this will be the ratio of electrostatic force to gravitational force for an electron and a proton at any distance (taking the ratio before entering numerical values shows that the distance cancels). This ratio gives some indication of just how much larger the Coulomb forcefulness is than the gravitational force between two of the near common particles in nature.
As the instance implies, gravitational force is completely negligible on a small scale, where the interactions of individual charged particles are important. On a large scale, such as between the Earth and a person, the reverse is true. Virtually objects are nearly electrically neutral, and and then attractive and repulsive Coulomb forces almost cancel. Gravitational force on a big scale dominates interactions betwixt large objects because it is always bonny, while Coulomb forces tend to cancel.
Section Summary
- Frenchman Charles Coulomb was the first to publish the mathematical equation that describes the electrostatic force between two objects.
- Coulomb's law gives the magnitude of the force between point charges. It is
, where q one and q 2 are two point charges separated by a distance r, and - This Coulomb forcefulness is extremely basic, since nearly charges are due to indicate-like particles. It is responsible for all electrostatic effects and underlies about macroscopic forces.
- The Coulomb forcefulness is extraordinarily stiff compared with the gravitational forcefulness, another basic strength—merely unlike gravitational force information technology tin can cancel, since it can be either attractive or repulsive.
- The electrostatic force between ii subatomic particles is far greater than the gravitational forcefulness betwixt the same ii particles.
Conceptual Questions
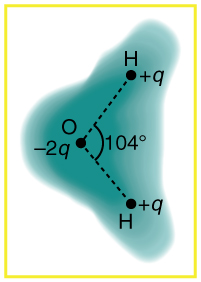 Figure 3. Schematic representation of the outer electron deject of a neutral h2o molecule.
Figure 3. Schematic representation of the outer electron deject of a neutral h2o molecule.
Use Figure 3 as a reference in the following questions. Effigy iii shows a schematic representation of the outer electron cloud of a neutral water molecule. The electrons spend more time virtually the oxygen than the hydrogens, giving a permanent charge separation as shown. Water is thus a polar molecule. Information technology is more easily affected by electrostatic forces than molecules with uniform charge distributions.
- Effigy 3 shows the accuse distribution in a water molecule, which is called a polar molecule because it has an inherent separation of accuse. Given water'southward polar graphic symbol, explain what effect humidity has on removing excess charge from objects.
- Using Effigy iii, explain, in terms of Coulomb's constabulary, why a polar molecule (such as in Effigy 3) is attracted by both positive and negative charges.
- Given the polar character of h2o molecules, explain how ions in the air form nucleation centers for pelting droplets.
Problems & Exercises
- What is the repulsive force betwixt two pith assurance that are eight.00 cm apart and have equal charges of –30.0 nC?
- (a) How stiff is the attractive forcefulness betwixt a drinking glass rod with a 0.700 μC accuse and a silk material with a –0.600 μC charge, which are 12.0 cm autonomously, using the approximation that they human activity like point charges? (b) Talk over how the answer to this problem might be affected if the charges are distributed over some area and do non human activity like point charges.
- Two point charges exert a five.00 N force on each other. What will the force become if the altitude betwixt them is increased by a factor of 3?
- Two point charges are brought closer together, increasing the force betwixt them past a cistron of 25. By what factor was their separation decreased?
- How far apart must ii point charges of 75.0 nC (typical of static electricity) exist to have a force of i.00 N betwixt them?
- If two equal charges each of 1 C each are separated in air by a altitude of i km, what is the magnitude of the force acting between them? You lot will see that even at a altitude as large as ane km, the repulsive force is substantial considering ane C is a very pregnant corporeality of accuse.
- A examination charge of +ii μC is placed halfway betwixt a charge of +6 μC and some other of +4 μC separated by 10 cm. (a) What is the magnitude of the forcefulness on the test charge? (b) What is the management of this force (away from or toward the +6 μC accuse)?
- Blank free charges do not remain stationary when shut together. To illustrate this, calculate the acceleration of two isolated protons separated past 2.00 nm (a typical distance between gas atoms). Explicitly evidence how you lot follow the steps in the Problem-Solving Strategy for electrostatics.
- (a) By what factor must you change the distance between ii bespeak charges to change the strength between them past a cistron of 10? (b) Explain how the distance can either increase or decrease by this factor and still cause a factor of ten alter in the force.
- Suppose you take a total accuse q tot that you tin can split in any manner. Once split up, the separation altitude is fixed. How do you split the charge to achieve the greatest force?
- (a) Mutual transparent record becomes charged when pulled from a dispenser. If i piece is placed to a higher place another, the repulsive strength tin be peachy plenty to support the height piece's weight. Assuming equal point charges (only an approximation), summate the magnitude of the accuse if electrostatic force is bang-up plenty to support the weight of a 10.0 mg slice of tape held i.00 cm above another. (b) Discuss whether the magnitude of this accuse is consistent with what is typical of static electricity.
- (a) Find the ratio of the electrostatic to gravitational force betwixt ii electrons. (b) What is this ratio for two protons? (c) Why is the ratio different for electrons and protons?
- At what distance is the electrostatic force between two protons equal to the weight of one proton?
- A certain v cent coin contains v.00 thou of nickel. What fraction of the nickel atoms' electrons, removed and placed i.00 m higher up it, would back up the weight of this coin? The atomic mass of nickel is 58.7, and each nickel atom contains 28 electrons and 28 protons.
- (a) 2 signal charges totaling 8.00 µC exert a repulsive forcefulness of 0.150 Northward on one another when separated by 0.500 yard. What is the charge on each? (b) What is the accuse on each if the strength is attractive?
- Betoken charges of 5.00 µC and –3.00 µC are placed 0.250 g autonomously. (a) Where tin a third accuse be placed so that the internet force on it is nil? (b) What if both charges are positive?
- 2 point charges q i and q two are 3.00 m apart, and their total charge is 20 µC. (a) If the force of repulsion between them is 0.075N, what are magnitudes of the 2 charges? (b) If one charge attracts the other with a forcefulness of 0.525N, what are the magnitudes of the two charges? Note that you may need to solve a quadratic equation to attain your answer.
Glossary
Coulomb's constabulary: the mathematical equation calculating the electrostatic force vector betwixt ii charged particles
Coulomb forcefulness: some other term for the electrostatic strength
electrostatic force: the amount and direction of attraction or repulsion between two charged bodies
Selected Solutions to Problems & Exercises
2. (a) 0.263 North; (b) If the charges are distributed over some area, there volition be a concentration of charge forth the side closest to the oppositely charged object. This effect will increment the net force.
4. The separation decreased by a factor of 5.
8.
ix. (a) three.2; (b) If the altitude increases by iii.2, then the strength volition decrease by a factor of 10 ; if the distance decreases past 3.ii, then the force will increase by a factor of 10. Either mode, the forcefulness changes by a cistron of ten.
11. (a) 1.04 × ten−9 C; (b) This charge is approximately 1 nC, which is consistent with the magnitude of accuse typical for static electricity
xiv. one.02×10−xi
16. (a) 0.859 g beyond negative charge on line connecting two charges; (b) 0.109 m from lesser accuse on line connecting ii charges
Licenses and Attributions
Source: https://www.coursehero.com/study-guides/austincc-physics2/18-3-coulombs-law/

0 Response to "Funny Video Related to Coulombs Law and Newtons Law"
Post a Comment